Physics A: Problem Set 24: Atomic spectra
recommended reading
| High Marks: | 5:8–5:10 |
| Barron's Let's Review: | 13.5 Models of the atom |
| physics.info: | n/a |
| Wikipedia: | Bohr model, Energy level |
| Khan Academy: | Bohr's model of the hydrogen atom |
| YouTube: | Why is glass transparent? |
| Mr. Machado: | 02 Spectra and Energy Level Transitions |
sample problem
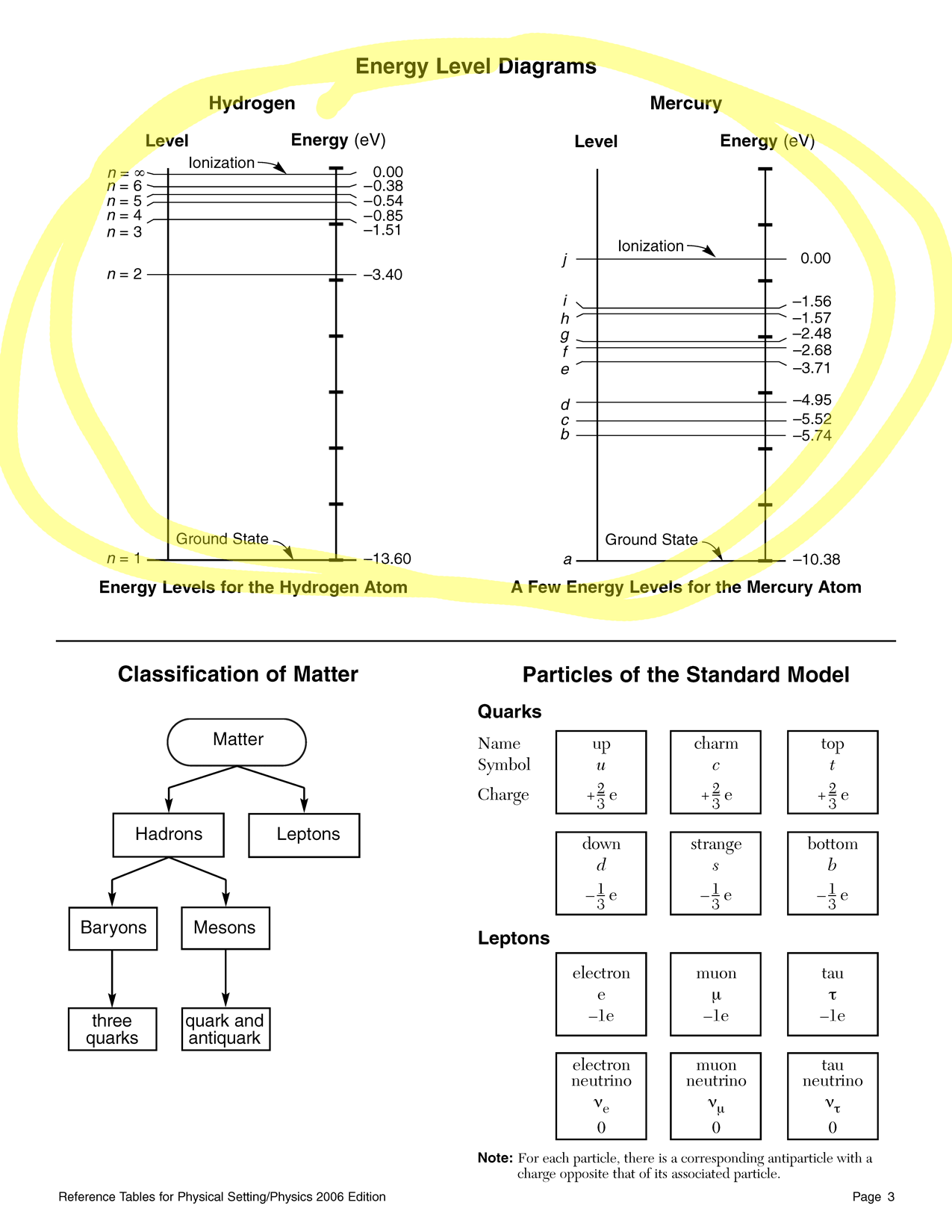
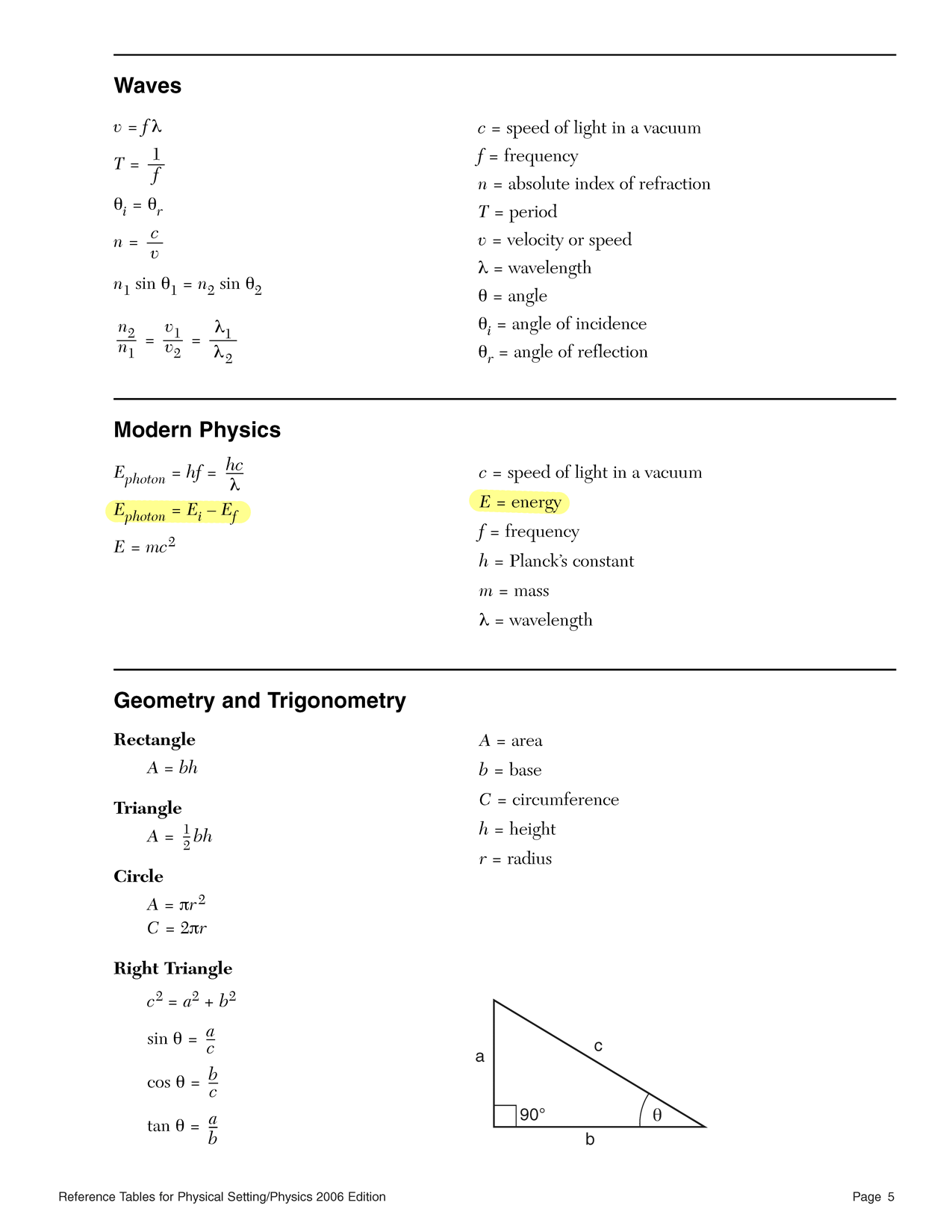
- In a mercury atom, as an electron moves from energy level i to energy level a, a single photon is emitted.
- Determine the emitted photon's energy in…
- electronvolts
- joules
- Determine the emitted photon's…
- frequency
- wavelength
- What kind of electromagnetic radiation is this?
- Determine the emitted photon's energy in…
homework
- An excited electron in a hydrogen atom falls from the n = 6 energy level to the n = 3 energy level. Determine the following quantities for the emitted photon:
- its energy in electronvolts
- its energy in joules
- its frequency in hertz
- its wavelength in meters
- its momentum in kilogram meters per second
- its classification in the electromagnetic spectrum